GALLIUM AND INDIUM METALS RECOVERY
FROM JAROSITE RESIDUES
By: Adolfo Rios Pita Giurfa
In the process for the obtention of electrolitic zinc at the zinc refinery of Cajamarquilla(Lima-Perú),leach solutions are purified in several steps,one of these is for the precipitation of Fe+3 as Jarosite(basic ammonium ferric sulphate). Together with Iron, Gallium and Indium precipitate,also as ammonium complexes.
This residue has the following assay:
Total Zn : 6.5%
Ca : 2.55%
Soluble Zn : 1.8%
Mn : 0.56%
Fe : 28.5%
Ni(ppm): 21
Pb : 2.2%
Co(ppm): 29
Cd : 0.03%
Tl(ppm): 54
Cu : 0.04%
K : 0.10%
Ag(oz/st) : 6.50
Na : 0.04%
Ga(ppm) : 320
Mg : 0.018%
In(ppm) : 580
Si : 0.85%
As : 0.056%
S/S: 0.03%
Sb : 0.06%
S/SO4: 11.78%
Al : 0.38%
Cl : 0.14%
Cr(ppm) : 90
Te(ppm): 22
Au(oz/st) : 0.014
Ge(ppm) : 8
In 1987 we began running tests having in perspective the gallium and indium recovery from the jarosite residue. In these preliminary tests we got a precipitate which contained about 10 times the amount found in the jarosite head.
From these preliminary tests we have an approximate idea of the eventual recovery process:
1)Acid wash(removal of soluble zinc)
2)Acid leach.
3)Copper precipitation at pH=~1.0 using Na2S
4)Obtention of a precipitate at pH=~2.5 using Na2S
5)Leaching of the precipitate.
6)Indium and gallium solvent extraction.
7)Gallium separation,extraction and electrodeposition in alkaline solution.
8)Indium cementation,melting to anode and electrorefining.
As nowadays there is a growing need in the electronic industry for these elements ( for the manufacture of gallium-indium arsenide computer chips), and being the continuous production of Jarosite residue a big problem for stockpiling and disposal, the project for its exploitation is of great importance.
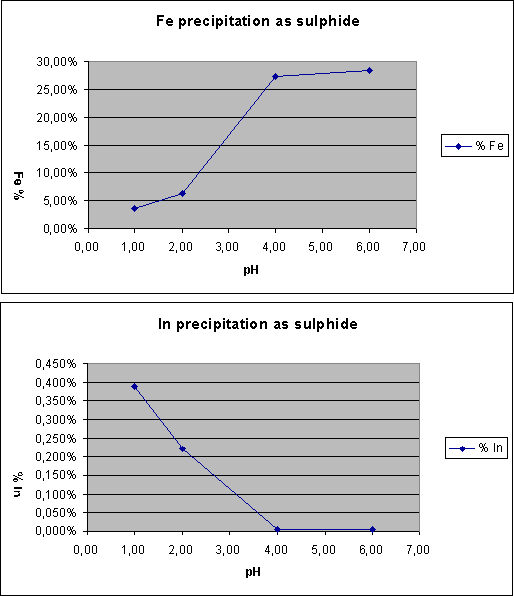
Gallium and Indium have similar properties, and both were separated from iron in the sulphide precipitate as the following graph shows: